The Nitrogen Cycle in Aquariums: Understanding the Fundamentals
Author: Vejay Anand
In the natural habitats of most common tropical fish, harmful nitrogen-containing compounds are relatively uncommon. Yet, in the confines of an aquarium, factors like overfeeding and overcrowding create an environment where nitrogen pollution can harm or even kill the fish. Understanding the essential compounds and processes of the nitrogen cycle is crucial.
The Nitrogen Cycle:
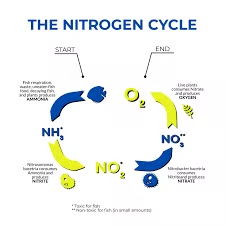
Nitrogen cycles from the air to plants, animals, and bacteria in a natural ecosystem before returning to the atmosphere. This intricate system requires no human intervention. However, in an aquarium, the nitrogen process differs. It's more of a biochemical cascade involving the continuous breakdown of nitrogenous compounds, starting with ammonia, progressing to nitrite, and culminating in nitrate. Aquarium plants absorb the final nitrates, or other methods are used to remove them from the water.
Cascade of Nitrogen Processing:
This cascade mimics the natural breakdown of waste in water within natural ecosystems. Even in a closed aquarium system, it's the responsibility of the hobbyist to establish and maintain this cascade. Ammonia, nitrite, and nitrate are the primary biological toxins that can develop in an aquarium, so the nitrogen cycle is essential to convert and remove these waste byproducts effectively.
This cascade takes time to establish in a live aquarium, often up to three months before a new aquarium can fully convert waste into nitrate. Gradually introducing younger, smaller fish allows nitrogen-converting bacteria to grow and keep pace with the rising waste levels.
Ammonia:
Bacteria swiftly convert fish urea and proteins into ammonia. In normal conditions, ammonia is a colorless, toxic gas. Elevated ammonia levels typically indicate overstocking or overfeeding. Nitrogen-fixing bacteria then oxidize this ammonia, converting it into nitrite.
Nitrite:
Nitrites pose a significant threat to aquarium fish. They result from the partial oxidation of ammonium ions. Nitrite-loving bacteria then transform nitrite into nitrate, rendering it less harmful. Preventing nitrite buildup involves:
- Controlled feeding.
- Regular partial water changes with well-aged water.
- Maintaining an appropriate animal population.
Nitrate:
Nitrates are the end product of nitrogen compound oxidation in the aquarium. They mainly stem from the breakdown of animal proteins, ammonium compounds, urine, excrement, food residues, deceased fish, snails, and plant leaves. While most freshwater tropical fish tolerate substantial nitrate levels, minimizing buildup through controlled feeding and population management is wise.
Plants:
Aquatic plants are crucial in reducing nitrate levels in a well-balanced aquarium. Similar to natural ecosystems, these plants actively utilize nitrates. In an unplanted tank, it falls on the aquarium owner to manage nitrate removal, typically at the final stage of the cascade.

